Critics of medical cannabis use often bring up the association between cannabis use and mental illness. In this context, the implication is almost always that cannabis use is unsafe and can exacerbate or initiate psychotic symptoms in both healthy and afflicted individuals. Yikes. While some of these claims are accurate, they are also incredibly misleading to the discussion about medical cannabis’ relationship with psychotic disorders. For example, it would be entirely factual to say that automobiles are involved in thousands of deaths each year. It would also be entirely factual to say that without automobile usage, vehicular death would not occur! This is clearly a very silly way to look at the situation: Automobiles transport food and medicines, and some automobiles like ambulances, literally save lives by allowing people to be rushed to proper medical facilities. The safety of a vehicle inevitably depends on who is driving it and what rules and road systems they are subject to. In this case it would be difficult to successfully argue that refusing to ride in an automobile is a healthy decision in modern society. Stating the facts without stating the whole context can be completely misleading.
Likewise, the relationship between cannabis and schizophrenia cannot be stated in one sentence. Both chemical and natural psychedelic substances, like cannabis, are well documented to trigger cases of mental illness. By transporting users to different mental states, these drugs can set off acute and long-term psychotic episodes, so any responsible medical doctor should advise patients with a history of mental illness or family history of mental illness to be careful with or completely refrain from psychedelic use. However, the underlying association here is very interesting – the endocannabinoid system is somehow involved in the same mental processes that regulate psychotic illness. Studies have shown that schizophrenic patients have higher densities of cannabinoid receptors and also higher levels of the body’s own version of THC, anandamide. As readers know from previous articles, the endocannabinoid system is not an off/on lightswitch with one effect and one activation. The endocannabinoid system consists of multiple pathways, including the CB1 receptor, the CB2 receptor, and allosteric binding without a traditional cannabinoid receptor at all! Some cannabinoids can bond to other types of receptors in the body and also partially activate and deactivate receptors. If this system is involved in the development of psychotic illness, and if we can both trigger and block various signaling pathways, then it seems obvious that some sort of manipulation of the system could actually help patients suffering from schizophrenia. As it turns out, this assumption is correct. Readers may know that while THC is primarily responsible for the psychedelic effects of cannabis, CBD can attenuate and dampen those effects. We’ve written before about CBD use as an anti-psychotic, but here we will evaluate new evidence specific to schizophrenia.
In 2012, researchers at the Federal University of Sao Paulo in Brazil began to test the effects of CBD on animal models of psychosis. Since it is difficult and sometimes unethical to test new mental illness medications on humans, researchers use animal models to focus on potential cures that can then be translated to humans. In this case, the researchers used spontaneously hypertensive rats (SHR) as a model of animal schizophrenia. “Spontaneously hypertensive” essentially means that the rats constantly have high blood pressure, which makes them a great model for many types of heart disease. However, recent research has also shown that these rats can also be used to study emotional processing impairment, which is one of the symptoms of schizophrenia.
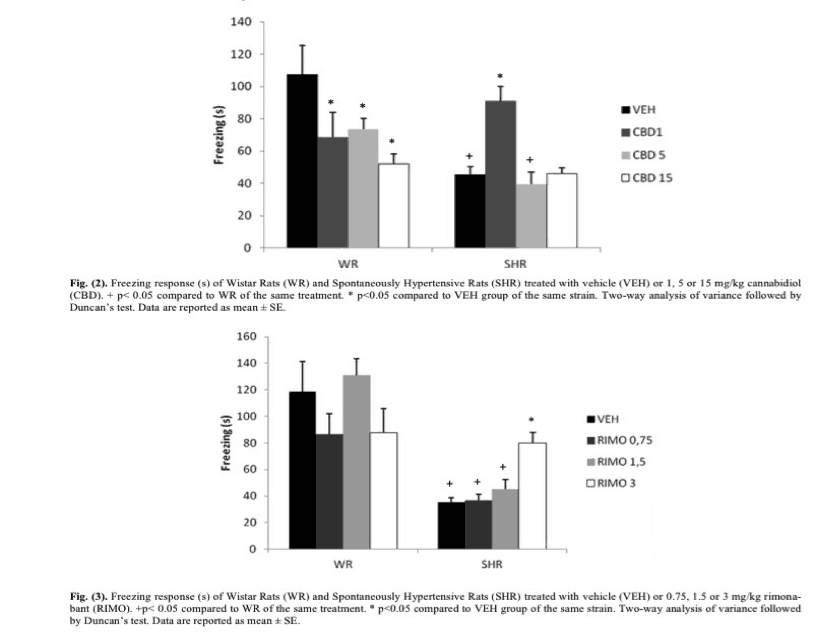
In this study, researchers used normally developing rats as a comparison to the SHR “schizophrenia” rats, and each rat was either given either an empty “control” dose or 1, 5, or 15 mg/kg of CBD respectively. Mg/kg simply means that the dose for each rat was adjusted to be proportional to body weight, which is quite similar to dosing in human pharmaceuticals. Thirty minutes later, the animals were tested with a training session of a Contextual Fear Conditioning task (CFC). For those unfamiliar, CFC is simply conditioning a subject to fear a certain stimulus or environment. In this case, rodents were placed in a dark chamber where short bursts of electric shocks were applied every thirty seconds for an interval of five seconds each. These rodents were then removed after 150 seconds, at which point, they had easily learned to associate the chamber with the shock procedure. Twenty-four hours later, the animals were placed once again in the chamber, this time without any electric shock. Researchers then recorded the amount of time the rodents spent “freezing” or not moving at all, which is an indication of the fear experienced from being placed in the same chamber. Healthy rodents should naturally learn to protect themselves by exhibiting fear in the chamber, while SHR rodents have been recorded to display freezing time deficits, meaning that they freeze less often, indicating a lower degree of fear association with the shock chamber, which in turn, indicates a lower degree of emotional processing. The same research group then repeated this experiment, but instead of using CBD, they applied doses of Rimonabant, a pharmaceutical that totally blocks or shuts off the CB1 receptor, the signaling of which is thought to be mostly responsible for the psychedelic effects of cannabis.
The results are given below:
(Graph Insert: “Graph showing the freezing time for both healthy (WR) and spontaneously hypertensive rats (SHR) with various doses of CBD and Rimonabant.
As we can see from the first graph, SHR rodents have a freezing time of almost half that of normally developing rats when no dose (VEH) is given. Interestingly, doses of CBD seem to lower the freezing response of normally developing rats. However, 1 mg/kg of CBD in SHR rats actually seemed to almost totally restore the normal fear response time, indicating that a low dose of CBD could be used to overcome the stunted emotional processing seen in SHR rats.
Likewise, as seen in the second graph, while Rimonabant had a fairly constant effect at various doses in normally developing rats, for SHR rats, Rimonabant seemed to restore normal freezing response time. This effect increased as the dose increased.
This research indicates that either pathway could successfully be used to restore normal fear conditioning response times in animal models of schizophrenia. However, the use of Rimonabant comes with its own problems, affecting other areas of mental health, which might mean that treatment with CBD is more realistic as a target pathway. If this same trend should hold in human trials, CBD could actually be a wonderful therapeutic target for those suffering from schizophrenia. The idea that mental health patients should refrain from cannabis is a drastic oversimplification of the situation. High-CBD cannabis strains might actually be very helpful to patients, especially in low doses. As it turns out, many schizophrenic patients already frequently use cannabis.
Although critics of medical cannabis may cite this as evidence that cannabis may contribute to schizophrenia, the fact that many schizophrenics also exist that have never used cannabis hints that it is more likely these patients have learned to self-medicate their condition.
To return to the automobile metaphor, a car is no safer than the driver and the road conditions present. In the road of life, mental health patients and normally developing persons alike must drive with caution and respect for the substances they are using, being careful to observe their own individual reactions and discussing their treatment with qualified health professionals. Only time will tell if cannabis or a cannabis derived substance will be a successful treatment for those suffering from schizophrenia, but at the time being, CBD shows high potential when compared to other substances currently available.
Works Cited
Raquel Levin, Valeria Almeida, Fernanda Fiel Peres, et al. Antipsychotic Profile of Cannabidiol and Rimonabant in an Animal Model of Emotional Context Processing in Schizophrenia. (2012) Current Pharmaceutical Design 18:4960-4965.