The endocannabinoid system is one of the biggest sleeper hits of modern science. Despite going ignored until the early 90’s, we’re discovering more and more how vital and essential this system of naturally produced cannabinoids, their receptors, and their enzymes are. From breast cancer pathogenesis to arthritis and pain treatment to neurological issue development, the endocannabinoid system is involved. For this reason, it should come as no surprise to readers that the endocannabinoid system has recently been found to play a role in the pancreas. Specifically, cannabinoid receptor 1 (CB1) is expressed in beta cells in the pancreas. The primary role of these beta cells is to produce and store insulin, the hormone that reduces blood sugar concentration. Lack of insulin leads to diabetes, which brings a host of related health issues. In multiple studies, CB1 receptors have been found to be able to trigger cell death of these beta cells. Thus, cannabinoid receptor regulation is likely essential to the control and pathogenesis of diabetes, and researchers aim to create new treatments by understanding this signaling. Ideally, researchers could stop beta cell death, preserve natural insulin levels, and therefore prevent or cure diabetes, rather than just supplying the body with externally-produced insulin. Toward that end, a team of researchers from various universities in South Korea has released a new study aimed to identify the mechanism of cannabinoid-mediated cell death in the pancreas.
Before starting with animal studies, the team investigated the basic mechanics of cell death in a mouse pancreas cell line. In this phase, the researchers treated the cells with a solution of low concentration WIN55,212-2, a synthetic cannabinoid found to activate CB1 receptors in a fashion similar to THC. These treated cells began to die at a rate that was dose-dependent, as seen in the graph below:
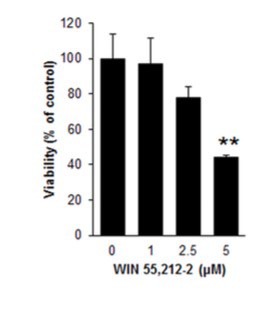
(Graph Inset: As amount of WIN55,212-2 increases, pancreas cell viability plummets.)
Interesting enough, when the chemical AM251 was applied, the effect disappeared. Because AM251 is a CB1 antagonist, which blocks the receptor, these two pieces of evidence confirm that the cell death must be mediated through the CB1 receptor. Researchers additionally noted through DNA content analysis that WIN55,212-2 lead to an increase in the proportion of cells in the G1 phase. This means that specifically the cells are stopping/dying during the G1 phase of cell duplication, the phase when cellular contents are duplicated in preparation for cell division.
Armed with specific knowledge about when and where CB1 receptors triggered cell death, the researchers then moved on to exploring how two molecules, cyclin D2 and Bcl-2, are involved. Researchers chose these molecules for investigation because they are pivotal to the growth and survival of pancreas beta cells. The hypothesis was that CB1 receptors somehow affect these molecules. Sure enough, when WIN55,212-2 was applied to cells, thus activating the CB1 receptor, both cyclin D2 and Bcl-2 decreased in a dose-dependent manner. This decrease causes beta cells to die.
Since this seemed to confirm the specific pathway through which CB1 receptors influence beta cells, researchers wanted to observe the two molecules live in action, through animal models. To do this, researchers created an animal model of diabetes by injecting mice with streptozotocin (STZ), a poison that selectively kills beta cells, then observed how the remaining beta cells recovered. However, while some of the mice were normal wild-type mice, others were CB1 receptor knockout mice, or mice genetically modified to live without CB1 receptors. These knockout mice were able to regenerate beta cells faster than the normal mice, indicating that the otherwise normal presence of the CB1 receptor may actually limit the re-growth of those cells. Likewise, when normally developing mice were injected with AM251 to block their CB1 receptors pharmaceutically, an almost identical rate of re-growth as those without any receptors in the first place was observed. Additionally, cyclin D2 and Bcl-2 increased as a result of the CB1 receptor blockade, once again confirming the role of that receptor in the death of pancreatic beta cells.
Thus, preliminarily, it seems like AM251 or any pharmaceutical means of disabling the CB1 receptor in the pancreas could allow beta cells to re-grow, thus limiting the development of diabetes. Unfortunately, disabling the CB1 receptor has serious consequences, as it is needed elsewhere in the body for a host of other healthy functions. For this to be a successful cure of diabetes, researchers must find a way to localize the effects of CB1 receptor antagonists. While this may be possible in animal models, it’s a little more difficult in humans where environmental conditions are constantly changing. However, the fact that the actual mechanism of the CB1 receptor-induced cell death is the down-regulation of growth factors cyclin D2 and Bcl-2 is important and worth noting here. Hypothetically, these molecules’ production could be up-regulated via another means, thus bypassing the body’s natural route for signaling beta cell growth. In other words, just because the endocannabinoid system naturally helps manage beta cell growth and death in the pancreas does not mean that the cure for diabetes has to be enacted that far upstream. As always, the more we understand about the endocannabinoid system, however, the more tools we have out our disposal for finding solutions to disease. To date, this may be one of the most hopeful avenues of diabetes cure research.
Works Cited
Jihye Kim, Kyung Jin Lee, Jung Seok Kim, et al. Cannabinoids Regulate Bcl-2 and Cyclin D2 Expression in Pancreatic Beta Cells. PLOS One (2016). DOI: 10.1371/journal.pone.0150981.

