Up to this point, we haven’t spoken much of the body’s own endocannabinoids. As a quick refresher, the endocannabinoid system consists of receptors and enzymes that metabolize any cannabinoids bonding to those receptors. However, our cannabinoid receptors do not exist for the purpose of consuming externally produced cannabinoids like those found in cannabis. These receptors exist because our bodies produce their own cannabinoids (endocannabinoids), which are used to signal and control various functions.
Of all our naturally produced cannabinoids, one of the most important is anandamide (AN). Anandamide, like our other endocannabinoids, is produced on the spot, where needed. It primarily bonds to CB1 receptors, giving it an effect profile similar to THC, the well-known psychoactive ingredient of cannabis. FAAH (fatty acid amide hydrolase) then metabolizes the anandamide, clearing the receptor for re-activation. Because anandamide is produced locally where needed, while external cannabinoids, like THC, are supplied to any tissue, comparing effect differences may be comparing apples to oranges. However, an oversimplification would be that ananadmide is the body’s own THC with a shorter effect endurance. In fact, the name “anadamide” is actually based on the Sanskrit word “ananda”, meaning “joy” or “bliss”. Rimonabant, the failed weight-loss drug we often reference on the blog, blocked CB1 receptors from being activated by anandamide. From known research, we can now safely guess that many of the negative psychological effects of Rimonabant resulted from preventing the body from using its own anandamide. In other words, anandamide seems to hold at least one key to mental health.
After its discovery in 1992, around the same time period as the discovery of the cannabinoid receptors themselves, researchers focused on understanding the ways that anandamide diffuses throughout the body. One report, from Virginia Commonwealth University (VCU) in 1997, administered externally produced ananadmide to mice through tail injections. At specific time intervals, researchers then harvested organs, breaking them down chemically and measuring the levels of anandamide in each tissue. This allowed the researchers to create tables and rudimentary plots of the time-related diffusion of externally injected anadamide through the body.
The results contained an interesting finding; anandamide diffuses into organs at different rates.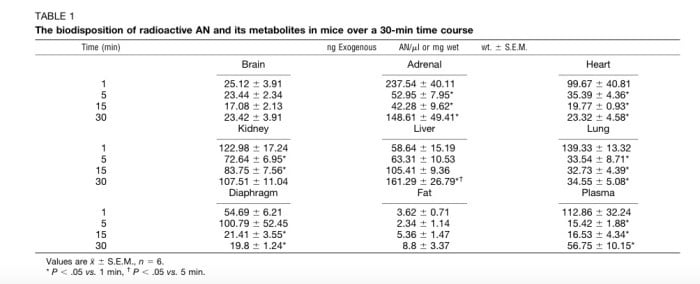
(Table showing the level of anandamide in various tissues as a function of time.)
For instance, while all tissues showed presence of anandamide within one minute (indicating a rapid onset), the brain showed little changes in levels from 1-30 minutes, while the adrenal gland spiked and then dropped to roughly 50% by the end of 30 minutes. Researchers concluded that this behavior could have less to do with the physical properties of absorption and more to do with the way some organs respond to anandamide by generating even more.
However, beyond merely testing levels of anandamide, visualizing the distribution in the brain is the next step to understanding the role it plays in our body. Up to this point, doing so has been difficult. Radiography is the most common technique for visualization, which relies on attaching radioactive markers to molecules being studied, then scanning the tissue for the presence of those markers. Unfortunately, there are limitations to radiography; in this case, radioactive markers would have be attached to the arachidonic acid component of anandamide. The attachment point would not normally be an issue, but arachidonic acid also happens to be a by-product of the metabolization of anandamide. Researchers noticed that the patterns of the anandamide distribution they were observing were highly similar to patterns of arachidonic acid diffusion. As a result, they suspected that the anandamide had broken down and that they were merely testing the diffusion of arachidonic acid resulting from anandamide’s metabolization.
Recently, researchers at Northeastern University found a way around this issue by applying an FAAH inhibitor at the same time as applying anadamide. Since FAAH, as mentioned above, is responsible for metabolizing anandamide, disabling it ensures that anadamide remains intact throughout its dispersion in the body, which then ensures that the radiograph is, in fact, displaying actual anandamide levels. Although this research has not yet been published, the results were presented at the ICRS 2015 Conference, so Cornerstone was able to get a sneak peak.
As seen in the graph below, anandamide takes a heterogeneous distribution, meaning that it clusters in a pattern rather than spreading uniformly over the brain. This pattern is distinct and exists with or without anandamide breakdown and also over the two different forms of anandamide shown in the left column. Simply put, we can be fairly confident that this is how externally applied anandamide is initially distributed in the brain.
(Graph displaying the radiographs of anandamide dispersion in two different forms and also with and without the FAAH inhibitor, URB597)
Of course, externally applied anandamide is still not the same as internally produced anandamide, and whether these distribution profiles match our body’s own natural distribution is unclear. Additionally, the effect anandamide causes in each component of the brain also remains unclear. Despite our relative sophistication in other receptor systems, researchers are still grappling with very basic questions about the function of the entire endocannabinoid system. In any case, readers can expect more articles from Cornerstone indicating anandamide’s role in the body as new information becomes available.
Works Cited
Kun Gian, Richard Duclos, and Samuel John Gatley. “Disposition and Metabolism of Anandamide in the Mouse Brain”. (2015) Presentation at ICRS 2015 Symposium.
Karen Willoughby, Sandra Moore, Billy Martin, and Earl Ellis. (1997) The Biodisposition and Metabolism of Anandamide in Mice. The Journal of Pharmacology and Experimental Therapeutics (1997) 282:243-247.