Have you ever had a bad situation made worse by your own reaction? Have you ever wished you had been slower to react or had reacted with less intensity and more deliberation? This also often occurs on a cellular level. What happens during brain trauma is a great example of the body’s reaction causing more problems than the injury itself.
Brain trauma is the leading cause of death in young people. Whether from injuries during rigorous or extreme activities such as sports, or from less avoidable car or workplace accidents, brain trauma can leave individuals with physical disability or worse, comatose. Researchers have also observed that beyond the acute (short-term) effects of brain trauma, individuals with previous brain injuries are at greater risk of developing Alzheimer’s and other neurodegenerative conditions. Countless studies have shown that one of the most critical factors in determining the long-term health consequences of head injury is the type and quality of treatment immediately surrounding the injury. In other words, the potential to change the outcome of the accident is greatest immediately after it has occurred, where small differences in timing and medical reaction can mean the difference between a healthy recovery or prolonged damage.
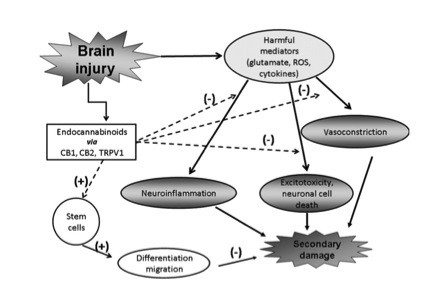
When such an injury occurs, the alarm bells of the brain start going off, launching the brain into an instinctually protective mode. The flow-chart found at the end of the article outlines the specific sequence of that process. During this protective behavior mode, the brain accomplishes several important physiological changes. These include shrinking blood vessels to decrease blood loss and controlling neural cell death. Another important change is that the brain seeks to protect itself from further injury by inflaming the area around the brain to form a natural cushioning. In some cases, this is useful to the body and can enable survival. However, the brain has limited ability to differentiate when and how much swelling is appropriate. As a result, the brain often swells too much. What happens then is that although the brain is attempting to protect itself from further pressure or force, it overswells to a point where it no longer safely fits within the skull. This leads to secondary injuries that are often much worse and can often lead to death. This is also the reason that head injuries should always be monitored closely, even when no problems appear at first. Although the individual may seem fine after the initial impact and may even walk away from the scene ready to continue work or activity, his/her brain will continue to swell and bleed. Thus what should have been a minor injury can be exacerbated to a life-threatening one by the brain’s own attempt to protect itself.
If you’ve been reading the Cornerstone blog, you may already suspect the role cannabinoids have been found to play in head trauma. Cannabinoids (and some terpenoids present in cannabis) are known anti-inflammatory agents, and this property seems to be the root of a large bulk of the medicinal properties of cannabis. The Institute for Drug Research in Israel recently published a review synthesizing the research surrounding cannabinoids and head trauma and adding the results of their own experimentation. In this review, they posited the idea that the cannabinoid potential for alleviating brain injury rests in the way with which the body already uses its own endocannabinoids to modulate injury. The researchers also divided beneficial effects of cannabinoids into the following major areas, which are summarized in the chart below: vasoconstriction, neuroinflammation, excitotoxicity, and stem cells.
As modulators of blood vessels (vasomodulators), endocannabinoids have been shown to cause hypotension, or decrease in blood pressure. The endocannabinoid 2-AG (2-Arachidonoyl-glycerol) is the brain’s most common endocannabinoid and is released into the blood at the same time as nitric oxide. Nitric oxide is one of the most effective vascular relaxing agents, so is it speculated that this pathway along with other non-nitric oxide pathways are responsible for the grand effect of relaxing blood vessels. This helps the brain restore normal blood flow.
In inflammatory models, endocannabinoids have shown the ability,to reduce inflammation in several ways. In one study that used over-stimulated macrophages (a type of immune-system white blood cell) as a cell model of inflammation, 2-AG reduced the amount of inflammatory material released, such as ROS (Reactive Oxygen Species). However, the Institute of Drug Research took this one step further by testing both levels of endogenous cannabinoids in rodents after injury, as well as the effect of adding external cannabinoids. By using a mouse model of closed head injury, they found that 2-AG increased to ten times its baseline within four hours of injury. This showed that the body already reacts to trauma by releasing its own cannabinoids. The group then found that adding more synthetic 2-AG resulted in lower fluid retention and lower swelling volume.
Another novel endocannabinoid, N-arachidonoyl-L-serine, has been shown to aid the pathway of neural stem cells in re-building areas of the brain. After adding it to cultures of neural precursor cells (NPC’s – a type of stem cell), researchers noted increased cell migration, which if duplicated in the body would lead to faster and more effective brain repair.
Although the exact role in excitotoxicity is unknown, research currently suggests that 2-AG may inhibit glutamate release. Excess glutamate release causes unhealthy shifts in sodium, potassium, and calcium, which in turn disrupts cell function and can lead to death. More research is needed to determine to what degree endocannabinoids may inhibit glutamate release.
Endocannabinoids have already been shown to play a role in the body’s handling of trauma. However, they also seem to hold a key to pharmaceutically assisting and modifying trauma reactions. This development in turn will mean fewer unnecessary deaths from accident and injury, illustrating yet another way that cannabis may inform future medical treatment.
This flow chart outlines the pathway of secondary damage from brain injury and the modulation of the resulting outcome via endocannabinoids. Negative signs correspond to a decrease in the respective effect, whereas positive signs correspond to an increase
Works Cited
Esther Shohami, Ayelet Cohen-Yeshurun, Lital Magid, Merav Algali, and Raphael Mechoulam. (2011) Endocannabinoids and traumatic brain injury. British Journal of Pharmacology (2011) 163, 1402-1410. DOI: 10.1111/j.1476-5381.2011.01343.x