Out of all the effects that cannabis provides, the euphoric, up-lifting change in perspective is surely what the plant is most known for. Countless artists from Jimi Hendrix to Tom Petty have created odes to this particular quality and many people swear by cannabis use as a method of enhancing the mind’s perspective, relying on it for a productive shift in mood and outlook. The same qualities of cannabis make it ripe for use as a recreational substance, which is a double-edged sword for those that use cannabis to medicate. Although recreational proliferation helps establish that cannabis use is safe (much safer in fact than alcohol or caffeine), it also obscures health benefits being derived from the plant. The uplifting effect of cannabis use blurs the line between what is recreational and what is healing, which in turn asks the question, “is it possible to be both?” Unfortunately, few studies have rigorously tested cannabinoids, the family of chemicals found uniquely in cannabis, in regards to potential for use as formal anti-depressants. One particular study, lead by a team at the University of Mississippi, set out to specifically evaluate those potentials of the most common cannabinoids.
In the study, the following cannabinoids were selected: delta-9-THC (the principal psychoactive compound of cannabis), delta-8-THC, cannabidiol, cannabigerol, cannabichromene, and cannabinol. Since breeding efforts specifically target delta-9-THC, it exists abundantly in many cannabis strains and is easy to extract. Others like cannabigerol are rare and take larger quantities and more complex methods to extract. In the past this has discouraged further testing of those cannabinoids, but as new extraction methods develop, the process is becoming easier. It’s important to keep in mind that the quantities of each used in the study may not exist in a normal smoking or edible session and the study was more focused on finding cannabinoids that have pharmaceutical potential than proving smoking cannabis as a treatment for depression.
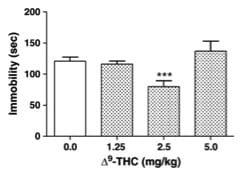
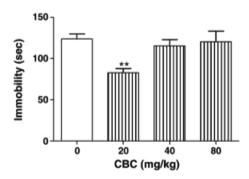
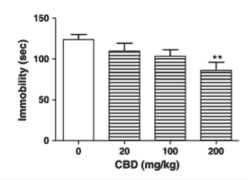
In the study, these cannabinoids were injected alone into mice, which were then observed under despair tests to measure reactions. The two specific tests chosen were the forced swim test and the tail suspension test, which are both established models of behavioral despair. Both procedures are thought to be good predictors of antidepressant actions, because many groups of therapeutically employed antidepressants show robust action in both tests. The forced swim test was the first test developed by the medical community and involves placing the mice in an inescapable situation, like a cylinder of water, are forced to fight to swim to stay afloat. Mice in this situation show behavioral despair within 4-6 minutes, which is seen in those mice decreasing mobility and “giving up”. Anti-depressant action is shown as a reduction in immobility duration and an increase in escape attempts like climbing. To be as objective about these measurements as possible, the group used a video camera and a computer to track the position and automate the scoring process. The tail suspension test was developed later as a companion to the forced swim test and is thought to be less strenuous or stressful than the forced swim test. This is less cruel and involves taping the mice by their tails to a rod suspended above a soft sponge (in case they manage to detach from the tape). Immobility is recorded over a time period and considered to be time period of hanging motionless. In this test, three independent raters counted the immobility times.
Several controls were used in the experiment to eliminate error. First, the traditional anti-depressants, Fluoxetine and Desipramine, were tested alongside, to give a standard of comparison for effect profile. A mostly saline solution (used as a control) was tested as well to control for any agitation of the mice from injection or handling. Immediately before testing, mice were subjected to locomotion tests using a chamber with multiple photo-beams to track movement. Without the locomotion tests, researchers could not rule out the cannabinoids had stimulant or depressant properties on locomotion, which would have affected performance in both the forced swim and the tail suspension tests, since those tests rely principally on motion.
As it turned out, several of the cannabinoids tested showed statistically significant anti-depressant properties. Delta-9-THC, and cannabichromene especially showed action in both tests, and cannabidiol (CBD) showed action in the more established forced swim test. None of these showed any affect on locomotion either as a stimulant or depressant, which made it clear that mobility effects were coming from anti-depressant properties specifically. Due to the anecdotal evidence about THC, this was not entirely surprising. However, the most interesting observation lies in the fact that both exhibited a dose-dependent response in the shape of a u-curve. What this means, is that a small amount of the substance may be strongly anti-depressive, a medium amount may have neutral effect, and a heavy amount may pick back up anti-depressant effect. The real dosage model may actually be more complicated and it’s important to remember that these drugs were being tested at higher than normal doses and only in three quantities. However, what is clear is that they are dose-dependent, something that individuals medicating with cannabis should take in mind. The dose may be just as important as the strain, and more is not always better.
Analyzing why these anti-depressant properties are occurring is currently beyond the scope of research. Researchers originally proposed that cannabinoids were achieving effects through CB1 receptors (the brain’s main receptors of cannabinoids). However cannbichromene does not affect CB1 receptors and still exhibited statistically significant anti-depressant properties. Moreover, research on endocannabinoids (natural cannabinoids found in the body) shows that both complete removal of CB1 receptors and activation of those receptors can lead to anti-depressant effects. While this contradiction may not mean that CB1 receptors are not involved, it does mean that finding the specific mechanism through which these cannabinoids are acting to achieve their effects will take time.
Abir T. El-Alfy, Kelly Ivey, Keisha Robinson, Safwat Ahmed, et. al. (2010) Antidepressant-like effect of delta-9-tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L, Pharmacology, Biochemistry, and Behavior 95 (2010) 434-442